|
|||||
|
|||||||
|
    An initiative of :Stichting Food-Info
|
| Food-Info.net> Topics > Food components > Food colours > Natural food colours ChlorophyllChlorophyll is a green pigment found in most plants, algae, and certain bacteria. As all leaves and thus all leafy vegetables contain chlorophyll, it is one of the oldest and most widely consumed pigments in our diet. As it has been in the human diet forever, it can be considered one of the most safe food components. Chlorophyll plays an important role in plants in the photosynthesis, the mechanism by which plants acquire energy.  Fig. 1: Chlorophyll, the green colour of plants (Source) Purified chlorophyll is used as a food colour with E-number E140, the more stable copper complexes of chlorophyll are number E141. StructureChemically chlorophyll is a mixture of several highly complex molecules, which consist of a ring structure (the porphyrin structure) with a central magnesium ion, and a long hydrophobic side chain (Fig 2.). Chlorophyll is produced through the same metabolic pathway as other pigments such as heme, the red colour of blood (which has an iron ion in the ring structure). There are a few different forms that occur naturally, which differ in some small changes in the ring structure, as well as in different side chains, see table 1. 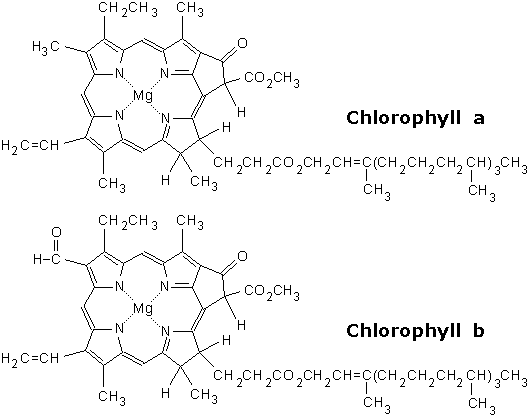 Fig. 2: Structure of the two most common chlorophylls
Table 1. Characteristics of natural chlorophylls
Chlorophyll is mainly extracted from alfalfa (Medicago sativa), nettles (Urtica dioica) or grasses. The chlorophyll can be extracted from the plants by several solvents, but has to be carried out rapidly in dim light to prevent degradation of the pigment. The resulting extract is further purified. Chlorophyll is not very stable, but stability can be increased by de-esterifying the chlorophyll and adding copper ions. These copper-complexes have a good green colour and are more stable than natural chlorophyll. UseDue to its safety, chlorophyll can be added unlimitedly to nearly all foods. However, as the colour is rather unstable, the applications are limited. Air, light, heat and pH all negatively influence the stability. Chlorophyll-coloured products should be dry and not exposed to light, air or high temperatures, In many cases the products are packed in dark packaging with a modified atmosphere to prevent chlorophyll degradation. For the copper complexes restrictions in use and dosage apply in different countries. This is mainly due to the toxicity of the copper, not the chlorophyll part of the complexes. |
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Food-Info.net is an initiative of Stichting Food-Info, The Netherlands | ||||||||||||||||||||||||||||||||||||||||||||||||||||||