|
|||||
|
|||||||
|
    An initiative of :Stichting Food-Info
|
| Food-Info.net> Topics > Food components > Carbohydrates StarchIntroductionStarch is one of the most abundant carbohydrates in nature and present in many food plants. Starch is by far the most consumed carbohydrates in the human diet. Traditional staple foods such as cereals, roots and tubers are the main source of dietary starch. Starch is one of the main energy sources in our diet. Chemically, starch is a polysaccharide consisting of a large number of glucose molecules. Starch has many applications, not only in the food industry. Starch and starch derivatives are widely used in paper manufacturing, in glues, such as wallpaper glue, cosmetics and even as a lubricant in oil drilling. Only the food applications are dealt with here. The word starch is derived from the Middle English sterchen, meaning to stiffen, which is appropriate since it can be used as a thickening agent when dissolved in water and heated.
Starch chemistryStarch is a mixture of two polymers of glucose and thus has the general chemical formula of (C6H10O5)n with n the number of glucose monomers. Starch molecules may reach a DP (Degree of Polymerisation) from a few hundred to many thousands of glucose monomers. The two polymers of starch are amylose and amylopectin. Depending on the plant, starch generally contains 20 to 25 percent amylose and 75 to 80 percent amylopectin. In general grain-derived starches have a higher amylose content as tuber-derived starches.
AmyloseAmylose is a linear polymer of glucose linked mainly by a(1?4) bonds, which promote the formation of a helix structure. It can be made of several thousand glucose units. Iodine molecules fit neatly inside the helical structure of amylose, hence, a common test for starch is to mix it with a small amount of yellow iodine solution. In the presence of amylose, a blue-black colour will be observed. The intensity of the colour can be tested with a colorimeter, using a red filter to discern the concentration of starch present in the solution. 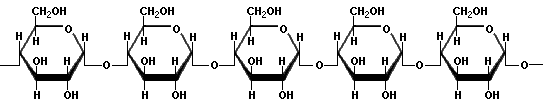
Structure of amylose (source)AmylopectinAmylopectin is a highly branched polymer of glucose. Glucose units are linked in a linear way with a(1?4) bonds. Branching takes place with a(1?6) bonds occurring every 24 to 30 glucose units. Amylopectin molecules may contain up to two million glucose units. 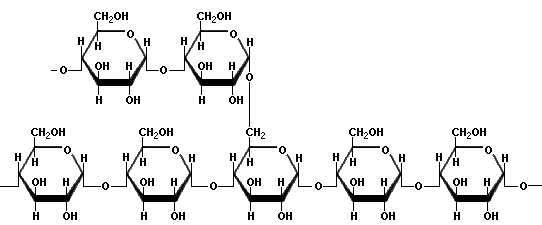 Simple amylopectin structure (source) Complex amylopectin structure, with each dot representing a glucose molecule. (source)Gelatinization and retrogradationGelatinization is the process in which starch becomes soluble, binds water and forms a gel. This process makes the starch more easily digestible. The use of starch as a thickening agent is based on this process. Starch is swelling up by heating and continues to swell up absorbing water and showing more viscosity and clarity along with increase of temperature. At a certain point the maximum viscosity has been reached. By further heating the starch molecules will move further apart and the viscosity is decreasing. Viscosity is gradually increasing again when the solution is cooled down and continuous cooling makes the solution cloudy. Leaving to stand the solution it will form a gel. The strength of the gel is determined by the type and concentration of starch in the product. When a starch gel is left to stand for some time, the amylose molecules will lose water and bind together. A similar process occurs when starch rich products, such as potatoes, will be stored for a long time. This process of recrystallisation of starch is called retrogradation.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
viscosity |
transparency |
stirring proof |
retrogradation |
potato |
very high |
clear |
low-medium |
medium |
maize |
medium |
opaque |
medium |
high |
wheat |
low-medium |
turbid |
medium |
high |
waxy maize |
medium-high |
clear |
low |
very low |
A modified food starch undergoes one or more chemical or physical modifications, which allow it to function properly under high heat and/or shear frequently encountered during food processing. The word 'modified' thus refers to the chemical structure of the starch molecule and has nothing to do with genetically modified plants or foods.
Food starches are typically used as thickeners and stabilizers in foods such as puddings, custards, soups, sauces, gravies, pie fillings, and salad dressings, but have many other uses. In many food products the conditions are not suitable for proper gelling of the starch. By chemically modifying the starch, the properties of the molecule change and the starch may be used in foods with for example low pH, or foods that can not be heated.
The modified starches are coded according to the International Numbering System for Food Additives (INS) and have the following numbers:
Number |
Modification |
Process limitations |
End-product specifications |
Dextrin roasted starch |
Dry heat treatment with hydrochloric acid or ortho-phosphoric acid |
Final pH 2.5-7.0 |
|
Acid treated starch |
Treatment with hydrochloric acid or ortho-phosphoric acid or sulphuric acid |
Final pH 4.8-7.0 |
|
Alkaline treated starch |
Treatment with sodium hydroxide or potassium hydroxide |
Final pH 5.0-7.5 |
|
Bleached starch |
Treatment with peracetic acid and/or hydrogen peroxide, or |
Added carbonyl group not more than 0.1% |
|
|
|
sodium hypochlorite or sodium chlorite, or |
No residual reagent |
|
|
sulphur dioxide or alternative permitted forms of sulphites, or |
Residual sulphur dioxide not more than 50 mg/kg |
|
|
potassium permanganate or ammonium persulphate |
Residual manganese not more than 50 mg/kg |
|
Enzyme-treated starch |
Treatment in an aqueous solution at a temperature below the gelatinization point with one or more food-grade amyolytic enzymes |
|
Oxidized starch |
Treatment with sodium hypochlorite |
Carboxyl groups not more than 1.1% |
|
Monostarch phosphate |
Esterification with ortho-phosphoric acid, or sodium or potassium ortho-phosphate, or sodium tripolyphosphate |
Phosphate calculated as phosphorus not more than 0.5% for potato or wheat, and not more than 0.4% for other starches |
|
Distarch phosphate |
Esterification with sodium trimetaphosphate or phosphorus oxychloride |
Phosphate calculated as phosphorus not more than 0.5% for potato and wheat, and not more than 0.4% for other starches |
|
Phosphated distarch phosphate |
Combination of treatments for Monostarch phosphate and Distarch phosphate |
Phosphate calculated as phosphorus not more than 0.5% for potato and wheat, and not more than 0.4% for other starches |
|
Acetylated distarch phosphate |
Esterification by sodium trimetaphosphate or phosphorus oxychloride combined with esterification by acetic anhydride or vinyl acetate |
Acetyl groups not more than 2.5%; phosphate calculated as phosphorus not more than 0.14% for potato and wheat, and 0.04% for other starches; and vinyl acetate not more than 0.1 mg/kg |
|
Starch acetate |
Esterification with acetic anhydride or vinyl acetate |
Acetyl groups not more than 2.5% |
|
Acetylated distarch adipate |
Esterification with acetic anhydride and adipic anhydride |
Acetyl groups not more than 2.5% and adipate groups not more than 0.135% |
|
Hydroxypropyl starch |
Esterification with propylene oxide |
Hydroxypropyl groups not more than 7.0%; propylene chlorohydrin not more than 1 mg/kg |
|
Hydroxypropyl distarch phosphate |
Esterification by sodium trimetaphosphate or phosphorus oxychloride combined with etherification by propylene oxide |
Hydroxypropyl groups not more than 7.0%; propylene chlorohydrin not more than 1 mg/kg; and residual phosphate calculated as phosphorus not more than 0.14% for potato and wheat, and not more than 0.04% for other starches |
|
Starch sodium octenylsuccinate |
Esterification by octenylsuccinic anhydride |
Octenylsuccinyl groups not more than 3%; and residual octenylsuccinic acid not more than 0.3% |
Starches are modified in several different ways that are shortly described below.
Cross linking improves process tolerance, which means the starch can withstand heating, acid ingredients, stirring, pumping and packing. This starch does not break down during cooking.
Stabilising helps the product resist retrogradation. This means the starch has good freeze/thaw stability and good stability over time.
Two other types of 'modified' starch are used in the food industry; pregelatinised starch and physically modified starch. These starches do not need to be labelled as “modified starch” on the food label.
They can be labelled “starch” as they do not differ chemically from natural starch.
Pregelatinised starch has already been cooked in water, gelatinised and then dried. It is also known as precooked starch, pregelled starch, instant starch, cold water starch and cold water swellable starch. When the powder is added to cold or warm liquids, it swells to form a gel and becomes viscous and does not need further heating. Pregelatinised starches can produce smooth or pulpy textures. The two textures are made using different drying methods. The smooth texture is used for soups and sauces. The pulpy texture is used in fruit pie fillings and tomato toppings for pizza.
Physically modified starch treated by the manufacturer without the use of chemicals. Starches can be physically modified by roll drying, extrusion, spray drying, and with heat and moisture treatment.
Physically modified starch is used for example in puddings and pies.
Resistant starch is starch that escapes digestion in the small intestine of healthy individuals.
Resistant starch has been categorized into four types:
Type of RS |
Description |
Food sources |
Resistance minimized by
|
RS1 |
Physically protected |
Whole- or partly milled grains and seeds, legumes
|
Milling, chewing |
RS2 |
Ungelatinized resistant granules, slowly hydrolyzed by a-amylase
|
Raw potatoes, green bananas, legumes, high amylose corn
|
Food processing and cooking |
RS3 |
Retrograded starch |
Cooked and cooled potatoes, bread, cornflakes, food products with repeated moist heat treatment
|
Processing conditions |
RS4 |
Chemically modified starches due to cross-linking with chemical reagents |
Foods in which modified starches have been used (for example, breads, cakes)
|
Less susceptible to digestibility in vitro |
Resistant starch is considered to be a dietary fibre, as it is not degraded in the small intestine and thus reaches the large intestine intact, where it is partially fermented.
Resistant starch is widely used in the baking industry as a fibre source in bread, cookies and similar products. Resistant starch can also be used as an ingredient that improves crispness in foods where high heat is applied to a product's surface during processing. It is also used in the production of extruded snacks.
Resistant starch formation is increased in a product during storage, especially at low temperatures. This is mainly due to retrogradation of the starch and thus of the type RS3.
Several health promoting properties have been described for resistant starch. It acts as a dietary fibre and is thus has a positive effect on the health of the large intestine. Resistant starch is completely degraded and fermented by the bacteria in the large intestine. These bacteria produce relatively high amounts of butyrate from resistant starch, and butyrate may prevent the formation of intestinal cancers. Resistant starch can also easily be used by diabetic patients as it has no effect on the blood glucose level. There are also indications that resistant starch has a positive effect on blood cholesterol levels and may prevent the formation of gall stones. Finally there are indications that resistant starch increases the calcium and iron absorption from the large intestine.
Starch can be hydrolyzed into simpler carbohydrates by acids, various enzymes, or a combination of the two. The extent of conversion is typically quantified by dextrose equivalency (DE), which is roughly the percentage of the glycoside bonds in starch that have been broken. Food products made in this way include:
Dextrins are produced by the degradation of both amylose and amylopectin. The result thus is a mixture of degradation product from both molecules. Different methods exist to degrade starch, such as by enzymes, acid or other chemicals. Industrial production is, in general, performed by acidic hydrolysis of potato or other starches. Dextrins have different applications and for each application a specific mixture is used. Maltodextrins are a class of smaller dextrins.
Dextrins are completely digested in the small intestine and provide the same amount of energy (calories) as glucose or sugar. They can not be used by diabetics. When produced from wheat starch, (malto)dextrins may still contain traces of gluten.
Cyclodextrins are a special class of circular dextrins produced by several bacteria. Cyclodextrins have many industrial applications, but are rarely used in foods.
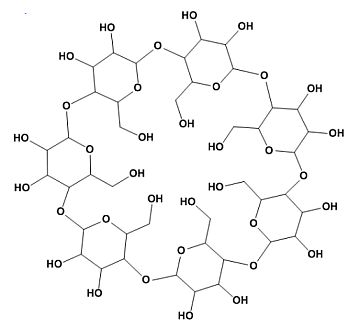
Corn or glucose syrups are solutions of highly degraded starch in water. In the US mainly corn starch is used, hence the use of corn syrup in the US or US produced foods. In other countries other starches may be used. The name glucose syrup would indicate a solution of pure glucose in water, but in practice it is a mixture of glucose, maltose en smaller maltodextrins.
Glucose syrups are widely used as a sweetener and structural agent in many foods. To increase the sweetness, glucose syrups can be treaded with the enzyme glucose isomerase to produce fructose, which is sweeter as glucose. These syrups are known as high fructose (corn) syrups (HFS or HFCS)
Like dextrins, the components of glucose syrups are digested in the small intestine and provide as much energy as glucose. They can not be used by diabetics. When produced from wheat starch, glucose syrups may still contain traces of gluten.
Starch is only found in plants, not in animals. Starch can be found in thousands of plant species where its main function is energy storage for the plant. High concentrations thus can be found in roots, bulbs, seeds and tubers of plants. All staple foods (wheat, corn, rice, potatoes, cassava etc) are rich in starch.
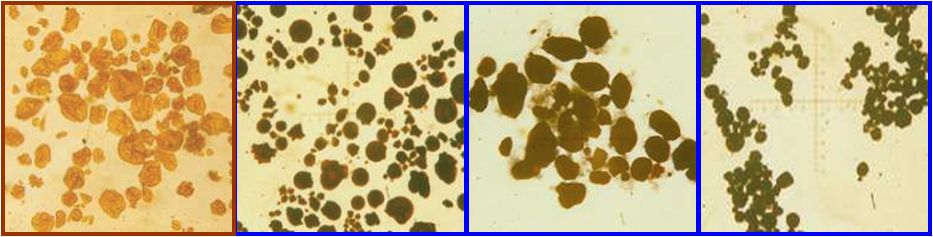
Without exception starch occurs in nature as granules. With the help of a microscope it is possible to determine the source of the starch, as the size and the shape of the starch granule differ for each kind of starch.
starch |
type |
size of grain in µm |
shape |
|
range |
average |
oval spherical |
||
potato |
tuber |
5 – 100 |
40 |
oval spherical |
maize |
grain |
2 - 30 |
15 |
round polygonal |
wheat |
grain |
1 - 45 |
25 |
round lenticular |
tapioca |
root |
4 - 35 |
25 |
oval truncate |
waxy maize |
grain |
3 - 26 |
15 |
round polygonal |
In relation to other starches, potato starch has a high moisture content. The tuber and root starches generally have a much lower fat content as the grain starches. These fats are predominantly glycerol fatty acid esters (maize) or phospholipids (wheat). The long hydrophobe (=apolar) chains of the fatty acid have a strong interaction with the starch molecule, which partly determines the properties of the starch. Starches with a high fat content, such as wheat starch, give turbid solutions and are generally less viscous than low-fat starches. Air oxidation of the fat gives these starches a rancid smell and taste, making these starches less suitable for the food industry.
The nitrogen content (proteins, oligopeptides, amides and amino acids) for potato starch and tapioca starch is also lower than that of the grain starches. The protein gives the typical floury smell to grain starches and is responsible for the tendency to foam formation. Protein in starch is unwanted, as by heating it will initiate Maillard reactions, which cause discoloration and flavour formation.
Starch in its basic refined form or as modified starch is used in cooking to thicken foods such as sauces and puddings. Starch thus is used in thousands of food products, besides the foods in which it is naturally present. Other uses include use as emulsifier, stabiliser, fat replacer, for better adhesion and as a clouding and glazing agent.
Starch is also used in the food industry in two different ways; one is in biodegradable and edible coatings and packaging materials, the other is as a mold in the preparation of confectionary. Gummed sweets such as jelly beans, liquorice and wine gums are not manufactured using a mold in the conventional sense. A tray is filled with starch and levelled. A positive mold is then pressed into the starch leaving an impression of 1000 or so jelly beans. The mix is then poured into the impressions and then put into a stove to set. This method greatly reduces the number of molds that must be manufactured. Starch is ideal for this application as it does not bind to the sweets and can be used indefinitely.
The main applications for starch, however, are outside the food industry. Starch is used as glue, for example for wallpaper, stamps or envelopes, as a lubricant in oil drilling, in the paper industry to make paper stronger, in pharmaceutical products as a filling agent, in baby diapers to increase the moisture absorption etc etc.
Sources and literature