Food-Info.net> Topics > Food components > Carbohydrates
Fructans
Introduction
Fructans are a large group of carbohydrates. Chemically fructans are polymers of fructose. They were first described by Rose, a German scientist, who found ďa peculiar substance from plan origin in a boiling water extract from Inula helenium" in 1804. This substance was named inulin by Thompson in 1818.
Fructan is an important and widespread non-structural carbohydrate that can be found in plants, algae and bacteria. Fructans are the dominant carbohydrate reserve in several families of the plant kingdom.
Fructans, especially inulin, are presently used in functional foods and as soluble fibres.
- Fructan chemistry
- Plant fructans
- Bacterial fructans
- Applications
Fructan chemistry
Fructans have a general structure of multiple fructose units, with or without a single glucose molecule at the beginning. In plants up to 1000 fructose units can be linked in a single fructan-molecule. Bacterial fructans can consist of up to 100.000 fructose units. The structure of fructans and fructan-derived oligosaccharides is generally abbreviated as GFx , with G indicating a glucose unit and Fx the number of fructose units. The number of glucose/fructose units is the Degree of Polymerisation (DP). Fructan derived oligosaccharides generally have a DP<10.
There are several types of fructans present in nature. These types are distinguished on the basis of the glycosidic linkages by which the fructose residues are linked to each other. They can broadly be divided into 3 groups; the inulin type, the levan type and the graminan type.
Inulins
The first group are the inulins, which are linear fructans, where the fructose units are (mostly) linked via a beta(2->1) bond. Nearly all fructans found in plants are of this type. The picture below shows 1-kestose. This is the shortest fructan of the inulin type. In larger fructans the coupling of fructose units is similar to the second fructose link to the first in the picture.
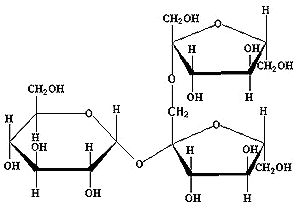
Fig 1: 1-kestose, the shortest inulin-type fructan (GF 2 )
Levans
The second group are the levans, which are also linear fructans, but where the fructose units are (mostly) linked via a beta(2->6) bond. This type of fructan is found in a many monocotyledon plants and in almost all bacterial fructans. The picture below shows 6-kestose. This is the shortest fructan of the levan type.
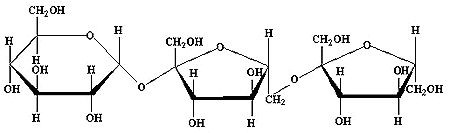
Fig 2: 6-kestose, the shortest levan-type fructan (GF 2 )
Graminian or mixed fructans
The third group is the fructans of the mixed type, which are also referred to as the graminan type. These fructans have both beta(2->1) and beta(2->6) linkage bonds between the fructose units, and thus contain branches. These fructans are less command and are found for example in grasses. The picture below shows 6,6&1 kestopentaose. This is one of the simplest fructans of this type.
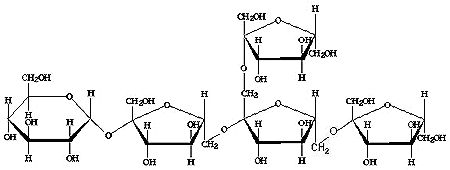
Fig 3: 6,6&1 kestopentaose, a short mixed type fructan (GF 4 )
Fructans in plants
Fructans are naturally produced by approximately 15% of the flowering plant species. From an evolutionary point of view fructans tend to be present within some of the most advanced families.
Important crops like wheat, barley, onion and chicory contain fructans. It has been estimated that as much as one third of the total vegetation on earth consists of plants that contain fructans.
In plants fructans are naturally present in the vacuole, in contradiction to starch which is stored in plastids (starch granules).
A large part of the fructan producing species is present in regions with seasonal drought or cold. It has been proposed that the function of fructan in plants could be the protection against drought as an osmoprotectant (protection against drying or salt) and cold for example as a cryoprotectant (protection against cold). A third function, storage of energy similar to starch, has not been proven, but may also play a role in some plant species...
Fructans are soluble in water and plants can easily change the length of the fructose chain. Together with the fact that fructans are present in the vacuole, this gives plants the opportunity to quickly change the length, and thus the number of molecules. More molecules mean that they can better withstand osmotic pressure or cold and thus these plants are more adapted to sudden changes in climate. Plants with fructans are therefore especially abundant in areas with seasonbound or sporadic rainfall in an annual cycle.
Plants that contain high concentrations of inulins include:
- Agave (Agave spp.)
- Burdock (Arctium lappa)
- Chicory (Cichorium intybus)
- Dandelion (Taraxacum officinale)
- Elecampane (Inula helenium)
- Garlic (Allium sativum)
- Jerusalem artichokes (Helianthus tuberosus)
- Jicama (Pachyrhizus erosus)
- Onion (Allium cepa)
- Wild Yam (Dioscorea spp.)
- Yacon (Smallanthus sonchifolius)


Fig 4: Yacon (left) and Jerusalem artichokes (right). (Sources 1, 2)
Fructans in bacteria
Some species from a number of bacterial genera have been reported to produce fructans. In the following genera fructan producing species are found: Tolypothrix, PseudomonasXanthomonas, Azotobacter, Erwinia, Streptococcus, Bacillus, Actinomyces, Rothia and Arthrobacter. Bacterial fructans are often much larger than fructans produced by plants. They can achieve a degree of polymerization of 100.000 or more fructose units. The structure of most of the fructans produced by bacteria is of the levan type.
Bacteria produce fructans as an energy storage, as a protective layer against outside stresses, or as an adhesion factor (glue). This latter is important in the formation of dental plaque, which consists for a large part of levan-type fructans produced by the oral bacteria on food-derived sugars.
Applications
Fructans are commercially mainly obtained from chicory roots (Cichorium intybus or the Jerusalem artichoke (Helianthus tuberosus) and of the inulin type. Degradation products of inulin are fructo-oligosaccharides. Fructo-oligosaccharides can also be produced enzymatically from sucrose by the enzyme fructosyltransferase.
Inulin is generally recognized as safe by the FDA in the USA and can be freely used as an ingredient worldwide.
Inulin is used increasingly in foods because it has unusual nutritional characteristics. It ranges from completely tasteless to subtly sweet and can be used to replace sugar, fat, and flour. Inulin can stabilise water into a creamy structure with the same mouthfeel as fat , which can be used as a low-calorie spread on bread.
Inulin is also used as a soluble colourless dietary fibre and used as such in many food products. A recent example is fibre-rich white bread, a type of inulin enriched bread, targeted for children that do not like 'brown' bread.
Fructo-oligosaccharides (FOS), are widely used as prebiotics, a class of functional foods. Inulin and FOS may also enhance the absorption of calcium and magnesium in the intestine.
Fructans can also be used as low calory sweeteners. Many short-chain fructans have a sweet taste, but can not be digested in the human intestine and thus provides much less energy as normal fructose.
Fructans are being applied outside the food industry, for example as (laundry) softener or biologically degradable plastics. Fructans can also be used as substrates in the production of ethanol or glycerol. Inulin is also used in medical tests to measure the total amount of extracellular volume and determine the function of the kidneys.
Inulin and FOS can not be used unlimited in foods, as they are rapidly fermented by intestinal bacteria. This fermentation results in the production of gasses and thus bloating, flatulence or diarrhea. The seriousness of these side effects depends on the type of fructan, the composition of the intestinal flora as well as the efficiency of the individual to absorb the gases. Some people report side effects after ingestion of 1 gram of fructans, whereas others have no side-effects with 40 grams.




